A look of sympathy tends to settle on many people’s faces when I tell them I study Chemistry. Most people seem to think that the subject involves lines going in every direction, symbols, and weird names that are all supposed to mean something. Now, I’m not going to lie, the subject is certainly not an easy one, nor is it lacking in jargon – in fact it’s quite the opposite, but contrary to popular belief it isn’t as mystifying as you might believe.
Is Chemistry logical?
In fact, the thing that really attracted me to the subject in the first place was its sheer logicality. This might seem bizarre when you delve into your Chemistry textbook and are greeted by what seem to be a series of random geometric shapes with names such as, 2,4-dihydroxyl-5-methylpyrmidine (which incidentally is the chemical name for thymine, a DNA base), but then you begin to realise everything (well, almost everything) follows a set of logical rules, or at least explanations. The naming of organic compounds, despite seeming daunting, comes down to a series of rather succinct rules. The 2 and the 4 merely indicate where the hydroxyl group (OH) are located on the carbon chain, whilst the “di” prefix simply reiterates the fact that there are two such groups. Though Chemistry has its exceptions, they are rather few and far between – something that isn’t true in Biology. Once you know the rules, it’s quite straightforward, and you can quite literally name any organic compound you want.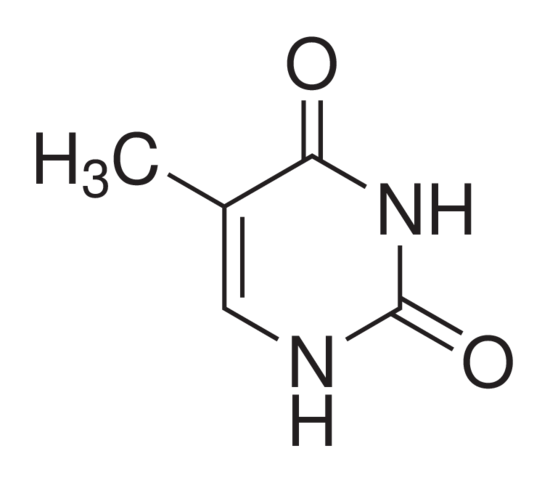

What can Chemistry explain?
But probably the thing I love the most about Chemistry is its ability to shed light on things you never really thought had an explanation. Take spicy food, for example. You know how your mum always told you to drink milk and not water when you’ve eaten something spicy? Well, Chemistry explains why this advice works. Capsaicin is the molecule responsible for the taste you detect as “spicy”, and it happens to be a non-polar molecule: a non-polar molecule is a molecule in which electrons are shared equally, i.e. there is no splitting of charge. Now there is a helpful saying we have in Chemistry that summarises solvation quite nicely: “like dissolves like”. By this we mean that polar substances dissolve in polar solvents, whilst non-polar substances dissolve in non-polar solvents. Now, water is a highly polarised molecule, with the oxygen atom pulling electron density towards itself and away from the two hydrogen atoms; this creates a partial negative charge around oxygen and a partial positive charge on the two hydrogens. Milk, on the other hand, though it also contains water, has a significant amount of fat. Fat, which is found in milk, is made up of fatty acid chains, and these saturated hydrocarbon chains are non-polar. Thus when you drink milk the capsaicin in your mouth interacts with these fatty acids, and thus dissolves.
This is just one of the sea of explanations that Chemistry provides to questions that might have plagued you for years. If such answers intrigue you as much as they do me, you might want to consider pursuing Chemistry. After all, they do say knowledge is power.


Sophie Z. is an undergraduate studying Environmental Chemistry at the University of Edinburgh. She is hoping to do her postgraduate work in Marine Chemistry.




